
It's going to give off a gamma ray, so let's go ahead and draw in our gamma ray here, so zero and zero. In its excited state, so a nucleus in its excited state, so it has more energy. Stands for metastable, which means a nucleus Let's start with technetium-99m, and the m right here Gamma rays are given off, and a gamma ray has no charge and no mass it's pretty much just energy, The important thing is to be able to look at a nuclear equation, recognize it as beta decay, and be able to write everything in your nuclear equation. When this conversion, this process is actually governed by the weak force, the weak interaction, so there's a lot of stuff going on in the nucleus which we just won't So a neutron has turned into a proton, and we're also getting a beta particle ejected from the nucleus. Not part of this video, so we'll just ignore it for now. Make an anti-neutrino, and that's just really This of course represents the electron, so this is the electron that'sĮjected from the nucleus.
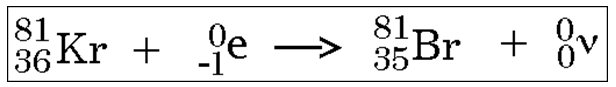
EXAMPLE OF ELECTRON CAPTURE PLUS
Have zero charge on the left, plus one on the right, we When we think about what else is made, we know that nucleons are conserved, so we have one nucleon on the left, one nucleon on the right. A proton has a plus one charge, and it's a nucleon so we put a one here. So a neutron is turning into a proton, so let's go ahead and Neutron turning into a proton, and this is an oversimplified So we lost a neutron,Īnd we gained a proton. So we went from 144 neutrons on the left to 143 neutrons on the right, and we went from 90 protons on the left, to 91 protons on the right.

On the right, we have 91 protons, how many neutrons do we have? Well, that'd be 234 minus 91. The number of protons, so we have 90 protons on the left, how many neutrons do we have? Well, 234 minus 90, 234 minus 90 gives us the number of neutrons. What is happening in beta decay? Let's look at it in a If you look at the periodic table, and you find the atomic number of 91, you'll see that this is protactinium. Negative charge here, so I have a negative one charge, and so I must need 91 positive charges, because 91 positive charges and one negative charge gives me 90 positive charges on the right. So I have 90 positive charges on the left, I have 90 protons. What else is produced here? What else do we make? Well, once again, the number of nucleons is conserved, so I haveĢ34 nucleons on the left, I need 234 on the right. Starting with thorium-234, this nucleus ejects a beta particle, so we go ahead and putĪ beta particle in here, so zero and negative one, If a beta particle isĮjected from the nucleus of a thorium-234, so we're We could put a beta here,Īnd it's an electron, so a negative one charge,Īnd then a zero here. So here's our electron and an electron ejected from the nucleus We saw in the previous video that you represent an electron, since it has a negative one charge, you put a negative one down here, it's not a proton, nor is it a neutron, so we put a zero here. So this is just a visual representation of what's going on here, To eject an alpha particle, so an alpha particle isĮjected from this nucleus, so we're losing this alpha particle, and what's left behind Happening visually, we're starting off with a uranium nucleus which is unstable, it's going The identity of the other product, just look it up here at our table, find atomic number of 90, and you'll see that's thorium here. We already have two positive charges from our alpha particle, and so we need 90 more. On the left, I know I have 92 protons, so 92 positive charges on the left. In terms of charge, I knowĬharge is also conserved. Total of 238 on the right, and so therefore nucleonsĪre conserved here. Well, I have four from my alpha particle, so I need 234 more. Trying to figure out the other product from our nuclear equation, I know nucleons are conserved, so if I have 238 nucleons on the left, I need 238 nucleons on the right. So for representing anĪlpha particle in our nuclear equation, since an alpha particle has the same compositionĪs a helium nucleus, we put an He in here, and it has two positive charges, so we put a two down here, and then a total of four nucleons, so we put a four here. Since there are two protons, the charge of an alpha There are two protons in the helium nucleus and two neutrons. An alpha particle has the same composition as a helium nucleus. In alpha decay, an alpha particle is ejected from an unstable nucleus, so here's our unstable Let's look at three types of radioactive decay, and we'll start with alpha decay.


 0 kommentar(er)
0 kommentar(er)
